|
The dynamics of oxidation and reduction reactions in transition-metal-cation-bearing aluminosilicate glasses and melts involves the coupled diffusion of electron holes (h.; polarons) and component network-modifying cations-a kinetic response orders-of-magnitude faster than any involving the diffusive motion of an oxygen species (ionic or atomic). The redox reaction causes a fundamental change of the amorphous molecular structure, with various results as a function of composition. For example, Fe2+-doped glasses devoid of alkali oxides, oxidation results in the homogeneous nucleation of a crystalline ferrite at an internal front; in melts, the ferrite forms at an internal front as well, in a process well-described as "isothermal undercooing." Reduction can result in the precipitation of colloidal iron metal at an internal front. Both oxidation and reduction reactions have specific morphological features at the free surface (Fig. 6).
There are a variety of applications for this melt/glass dynamics research, ranging from the crystalline nucleation of cooling magmas, to describing the thermodynamic potentials and kinetic processes involved in the formation of primitive chondrules, to discerning a unique way to internally heterogeneously nucleate inviscid ceramic melts.
Perhaps the most striking application is the modification of the float-glass process that was suggested by our understanding. "Float" processing is the pouring out of molten soda-lime silicate melt onto a bath of molten tin, where gravity, surface tension and mechanical deformation can be applied to produce very flat glass (automotive and architectural applications). Physically, the reaction between silicate and metal is a strong redox one, coupled with chemical interdiffusion. We have been able to model holistically the reaction (Fig. 7) based on the understanding outlined above, and with this model have been able to discern how to float-process distinctly more refractory glassmelts, i.e., increase the temperature of the float process while simultaneously managing the spatial extent of the diffusion-effected redox reaction. This process involves both the careful alloying of the metal float medium as well as the doping of the glassmelt to affect the molecular structure, and thus the mobilities of component ions.
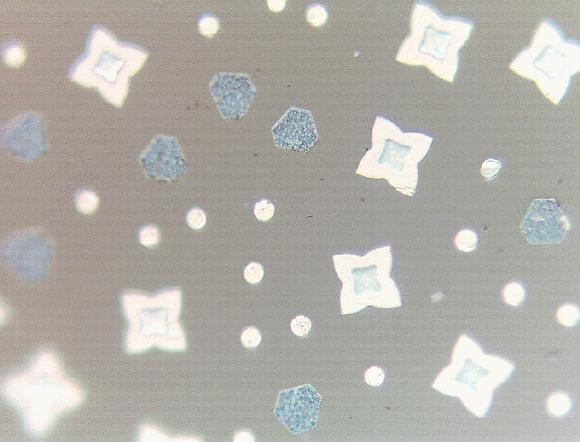
Figure 6: Free surface (reflected light image) of the free surface of a Fe2+-doped magnesium aluminosilicate melt droplet processed at 1300 deg C and an oxygen activity of 10-14, well below that where the FeO component of the melt is stable. Iron metal crystals form on the free surface and are coarsened by vapor-phase transport (Ostwald ripening). Most ion metal forms, however, as a nm-scale colloid at an internal reduction interface in a dynamic that includes inward diffusion of Mg2+ and Fe2+ and outward diffusion of h..
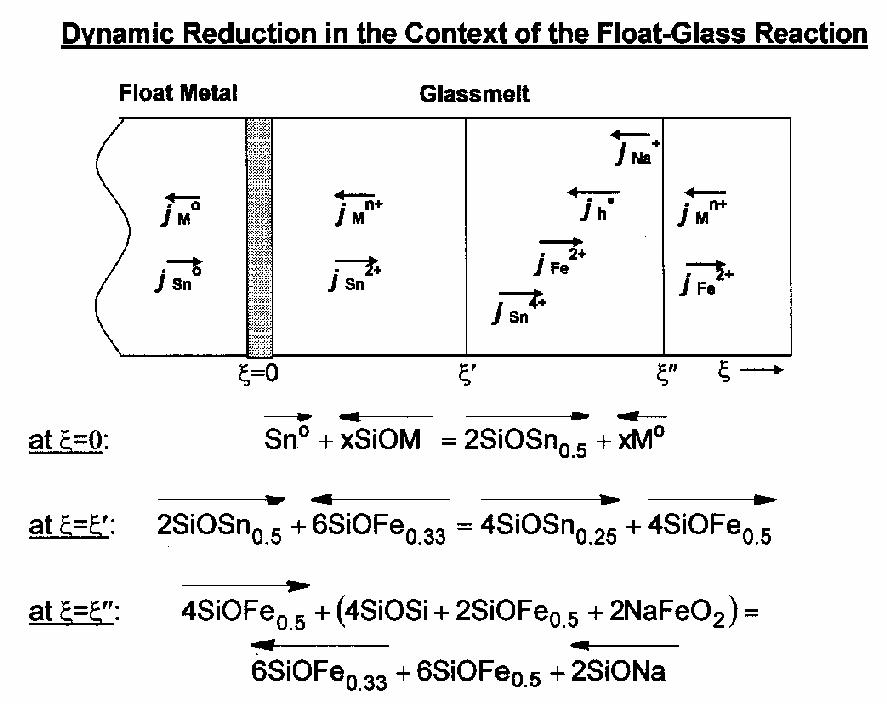
Figure 7: Reaction schematic for the float-glass process where a sodium aluminoborosilicate melt is in contact with a liquid gold-tin alloy. The dynamic involves (at x=0) oxidation of tin to Sn2+ in a redox couple with component ions of the glassmelt and its subsequent incorporation into the glassmelt (ion interdiffusion); (at x=x') oxidation of Sn2+ to Sn4+ by the acceptance of electron holes; (at x=x'') release of electron holes to diffuse outward in a local t\reduction reaction that changes the molecular structure of the glassmelt. The extent of reaction can be controlled through the combination of the useful alloying of the metal float medium (e.g., to lower the chemical activity of Sn) and the doping of the glassmelt to increase the concentration of h..
Sample Publications:
Cooper, R.F., J.B. Fanselow, J.K.R. Weber, D.R. Merkley and D.B. Poker (1996). Dynamics of oxidation of a Fe2+-bearing aluminosilicate (basaltic) melt. Science, 274, 1173-1176.Link to sciencemag website
Cook, G.B. and R.F. Cooper (2000). Iron concentration and the physical processes of dynamic oxidation in an alkaline earth aluminosilicate glass. Am. Mineral., 85, 397-406.Download pdf
Cook, G.B. and R.F. Cooper (1999). Redox dynamics in the float-processing of glasses I: Reaction between undoped and iron-doped borosilicate glassmelts and a gold-tin alloy. J. Non-Crys. Solids, 249, 210-227.Download pdf
|


